Dermatology for the Intersection of Skin and Technology
The U.S. leader in dermatology consulting for the skin–device interface, from wearables to medical devices
-
Smart watches
-
Fitness trackers
-
AR/VR goggles
-
Earbuds
-
Consumer electronics
-
Headphones
-
Fitness rings
-
Interstitial fluid sensors (CGM)
-
Electrical activity sensors (ECG, EMG)
-
Skin worn medical devices
-
Remote medical sensors
-
Hearing Aids
-
Microneedle array patches
-
Flexible electronics
BOHLD unites dermatology expertise, regulatory insight, and wearable device know-how to deliver actionable, evidence-based solutions —
in the lab, on patient skin, and in device design
SERVICES
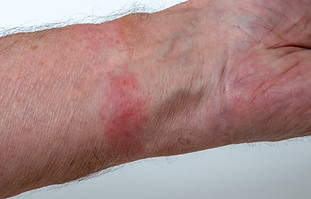
BOHLD offers board-certified dermatological expertise applied towards problem solving for any consumer or medical device that contacts the skin
-
Implement standardized workflows to capture relevant images and information about skin irritation events
-
Analyze user skin irritation complaints and images to determine underlying cause and how to prevent future occurrences
-
Critically review prototype device design and materials with the goal to minimize skin irritation risks
-
Generate "best practices" instructions for device users to minimize skin irritation risks
-
Develop protocols for remote wearable sensors that maximize signal collection while minimizing skin irritation
-
Identify roadblocks that limit the comfort and wearability of devices so that device use can grow in a sustainable manner.
-
Review extractable and leachable data to help identify and categorize potential allergens or irritants
-
Design and advise on skin allergy patch testing or provide interpretation of patching testing results
-
Training for designers, engineers, and legal teams to help them understand the skin issues that can arise with skin-worn devices. This training will help your team understand the potential risks associated with skin-worn devices and how to mitigate those risks
PROJECTS
BOHLD has over a decade of consulting experience in this field, working with industry leaders in technology, medical device companies, and small start-up companies. Our breadth of experience includes consultative work related to over thirty types of smart watches, fitness trackers, ear-worn audio devices, hearing aids, remote medical sensors, medical devices worn on the skin, AR/VR face-worn devices, and microneedle-arrays patches
Some Current Projects:
-
Liaison with safety and legal personnel to provide third-party, independent dermatologic medical evaluations
-
Contracted by a leading technology company to assess AR/VR head strap designs to optimize comfort and skin compatibility for users. BOHLD's evaluation focused on key factors such as material selection, weight distribution, and design ergonomics to prevent skin irritation and discomfort during prolonged use. Provided recommendations for incorporating breathable and thermoregulatory materials, adjustable strap lengths and additional tensions settings to enhance overall user experience while minimizing potential skin issues
-
Provided dermatological expertise to a remote medical sensor company to analyze and interpret post-market skin irritation data. Correlated reported irritations with skin preparation technique, device-use characteristics, and application methods. Formulated clear evidence-based conclusion for FDA regulatory documentation and recommended product safety enhancements
-
Training for engineers and designers at a leading consumer electronics company: Explored the critical concepts of biocompatibility and its importance in product design, focusing on the potential risks of allergic contact dermatitis (ACD) and irritant contact dermatitis (ICD) associated with materials or material properties used in consumer electronics. Additionally, discussed thermal contact burns (TCB), examining the clinical characteristics of different heat-related injuries to understand what a TCB from a wearable device might look like on the skin. The aims of training are to equip participants with the knowledge necessary to create safer, user-friendly products that prioritize consumer or patient health and well-being
PRODUCTS
BOHLD offers a range of specialized questionnaires tailored to gather essential clinical information for accurate diagnoses by credentialed dermatologists.
These questionnaires are crafted to create a highly structured environment, ensuring that inquiries are clear and unambiguous. They are designed for quantifiable data collection, enabling automated algorithmic analysis to establish thresholds for identifying cases of significant medical concern and, when appropriate, facilitating predictive diagnoses.
Accessible via cloud platforms, these questionnaires help automate processes and reduce manpower needs. The available formats cater to various device categories, including wrist-worn, ear-worn, face-worn, and medical devices worn on the skin.
Additionally, BOHLD offers custom questionnaire development to meet specific clinical needs

Why BOHLD?
Most wearable product development teams have incredible scientists, engineers, physicians, leaders and designers but lack dermatologic expertise. When devices cause skin irritation, there is lack of uniformed structured reporting, inconsistent terminology and haphazard collection of images. The net result is too little usable data to provide certainty about the underlying diagnosis
When device users develop uncomfortable skin irritation, there is dissatisfaction, loss of consumer confidence and potential legal and regulatory ramifications
BOHLD is a consultancy focused on providing dermatological expertise when questions arise regarding skin-worn devices. We are the dermatology experts that companies rely on when skin related complications or questions arise
Areas of Expertise
Wrist-Worn
Ear-Worn
Face-Worn
Skin-Worn Medical Devices




MicroNeedle Array